
Somnoplasty® Frequently Asked Questions
Haven't you suffered from snoring long enough?
SOMNOPLASTY®
A Simple Treatment for
Habitual Snoring
- What is the procedure? How does it work?
- Does the procedure hurt?
- What can I expect post-treatment?
- Does it work? Will I have to come back for additional treatments?
- Is this a permanent cure?
- Is the procedure approved?
- How many patients have been treated to date?
- What type of anesthesia is involved?
- How does this compare with other treatments currently available?
- What is the pre-treatment regimen?
- What are the risks? What are potential complications?
- How soon will I see results?
- What are the benefits?
- How do I know if I qualify for this procedure?
Contact Us to set up an Somnoplasty® evaluation today.
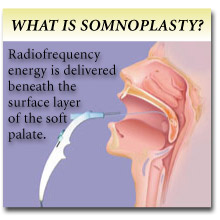
What is the procedure? How does it work?
Somnoplasty® uses low-power, low-temperature radio frequency energy to treat a well-defined area in the uvula or soft palate. Radio frequency energy is delivered beneath the surface layer of the soft palate. The treated tissue is heated just enough to kill the cells surrounding the electrode. Over the next six weeks to eight weeks, the treated tissue is naturally absorbed by the body, reducing the volume of the tissue and stiffening the soft palate area. Since the delicate surface of the palate is protected, the Somnoplasty® procedure causes minimal pain in most patients, and allows for a quick recovery.
Does the procedure hurt?
Prior to the Somnoplasty® procedure, your palate will be anesthetized. This is similar to having Novocaine when you go to the dentist. You should not feel pain during the Somnoplasty® procedure itself.
What can I expect post-treatment?
There may be some swelling and discomfort for a few days following the procedure, not unlike the feeling of an oncoming cold. During the next month or so you should experience a gradual decrease in your snoring. Depending on your level of snoring, the Somnoplasty® procedure may need to be repeated.
Does it work? Will I have to come back for additional treatments?
If most or all of your snoring originates from the palate, there is an 85% likelihood of success of the procedure in one or two treatments.
Is this a permanent cure?
Since this is a new procedure, long-term clinical data are not yet available.
Is the procedure approved?
The Somnoplasty® System was cleared by the U.S. Food and Drug Administration for use in the treatment of the palate in July 1997; December 1997 for turbinates and November 1998 for obstructed sleep apnea.
How many patients have been treated to date?
More than 6,000 patients have already been treated with the Somnoplasty® Procedure.
What type of anesthesia is involved?
The Somnoplasty® Procedure for the palate is performed under local anesthesia in an outpatient setting. The patient is not sedated. The procedure takes approximately 30 minutes, including 10 minutes necessary for the actual treatment of the palate.
How does this compare with other treatments currently available?
Non-surgical approaches include weight loss and other lifestyle modifications or the use of an oral appliance to reposition the jaw during sleep. A variety of traditional surgeries are also available to remove the excess tissue. For example, LAUP (Laser-Assisted Uvulopalatoplasty Procedure) removes tissue by laser at significantly higher temperatures than the Somnoplasty® procedure, and therefore has more post-operative pain.
UPPP (Uvulopalatophaaryngoplasty) is the surgical removal (under general anesthesia) of the uvula and a portion of the soft palate, expanding the airway. It is usually performed only on patients with sleep apnea in addition to snoring. The Somnoplasty® procedure gently reduces the tissue volume in the soft palate and uvula, and typically does so without the pain associated with conventional and laser-assisted surgeries.
What is the pre-treatment regimen?
It is recommended that you discontinue the use of aspirin or NSAIDS (Non-Sternoidal Anti-Inflammatory Drugs, i.e. ibuprofen, naproxen sodium) 10 days prior to the Somnoplasty® procedure.
What are the risks? What are potential complications?
Potential complications are the same as those associated with outpatient procedure requiring local anesthesia and may include: bleeding, infection, hematoma, injury to surrounding tissues/structures, swelling that may feel like something is in the throat. You may need to sleep with your head elevated at a 45 degree angle for 1-2 nights after the procedure to avoid difficulties breathing.
How soon will I see results?
You should experience a gradual decrease in your snoring during the six to ten weeks following your procedure.
What are the benefits?
The Somnoplasty® Procedure causes minimal pain in most patients, and allows for a quick recovery. The procedure for the palate is performed under local anesthesia in an outpatient setting. The patient is not sedated. The procedure takes approximately 30 minutes, including 10 minutes necessary for the actual treatment of the palate.
How do I know if I qualify for this procedure?
We would like to stress the importance of a proper diagnosis. If you believe you suffer from habitual snoring, and your level of snoring is disruptive to your personal life, we encourage you to come in for an evaluation. Contact Us for more info.
Copyright ©1999 Somnus Medical Technologies, Inc.
All Rights Reserved. Somnus, Somnoplasty, and the Somnus Medical Technologies logo are trademarks of Somnus Medical Technologies, Incorporated.

